Write the Complete Ground-state Electron Configuration of B
Therefore the expected electron configuration for Chromium will be 1s 2 2s 2 2p 6 3s 2 3p 4 4s 2 3d 9. The remaining electron will go in the 2p orbital.
Electron Configurations For The Second Period Video Khan Academy
Write the full ground-state electron configuration for each.

. Now the noble gas that comes. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 9. Write the complete ground-state electron configuration of arsenic 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p³ Draw the lewis structure of water H2O and then determine if.
When we write the configuration well put all 26 electrons in orbitals around the nucleus of the Iron atom. 1s2 2s2 2p6 3s2 3p6 3d10 4s1. A S2- b Rb b Au This is the initial text Question 37 V Saved 37.
Write a complete ground-state electron configuration for each of the following atoms or ions. Carbon He2s 2 2p 2. Neon He2s 2 2p 6.
First week only 499. After the 4s is full we put the remaining four electrons in the 3d orbital and end with 3d4. Write a complete ground-state electron configuration for each of the following atoms or ions.
Solution for Write the complete ground-state electron configuration of the atomion listed below. Aluminum Ne3s 2 3p 1. Write a complete ground-state electron configuration for each of the following atoms or ions.
Write the complete ground-state electron configuration of Ca². Electronic configuration of boron. Start your trial now.
In writing the electron configuration for Calcium the first two electrons will go in the 1s. In order to write the Iron electron configuration we first need to know the number of electrons for the Fe atom there are 26 electrons. Find step-by-step Chemistry solutions and your answer to the following textbook question.
Write the complete ground-state electron configuration for Al 3. Experts are tested by Chegg as specialists in their subject area. This means that the electron configuration of a neutral boron atom must account for a total of 5 electrons.
In this video we will write the electron configuration for B 3 the Boron ion. Fluorine He2s 2 2p 5. Fe Fe2 and Fe3 Electron.
Write the expandedcomplete ground state electron configuration of the element Strontium Sr Question. Write a complete ground-state electron configuration for each of the following atoms or ions. When we write the configuration well put all 20 electrons in orbitals around the nucleus of the Calcium atom.
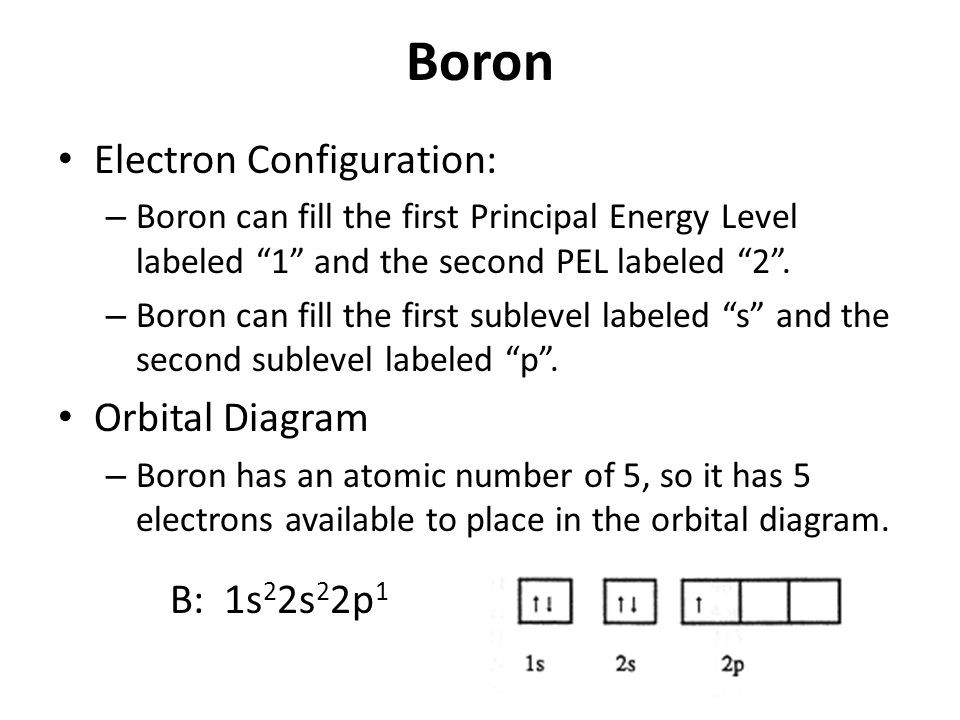
Boron is the fifth element with a total of 5 electrons. Once we have the configuration for Fe the ions are simple. Start by writing the complete electron configuration of boron B.
Note that when writing the electron configuration for an atom like Cr the 3d is usually written before the 4s. For the Cu ion we remove one electron from 4s1 leaving us with. Oxygen He2s 2 2p 4.
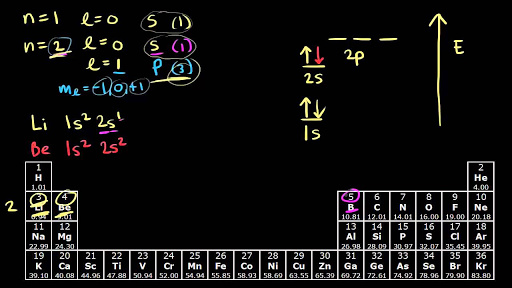
Boron is located in period 2 group 13 and has an atomic number equal to 5. In writing the electron configuration for Boron the first two electrons will go in the 1s orbital. This give us the correct configuration of.
Since 1s can only hold two electrons the next 2 electrons for B goes in the 2s orbital. Boron He2s 2 2p 1. In order to write the Calcium electron configuration we first need to know the number of electrons for the Ca atom there are 20 electrons.
Therefore the B electron configuration will be 1s 2 2s 2 2p 1. The Ca2ion is formed by the removal of two electrons from. 119 rows ELECTRON CONFIGURATION.
A Br b Mg c Se. We review their content and use your feedback to keep the quality high. Solution for Write the expandedcomplete ground state electron configuration of the element Strontium Sr close.
Write the complete ground-state electron configuration of aluminum. For the Cu2 ion we remove a total of two electrons one from the 4s1 and one form the 3d10 leaving us with. Well also look at why Boron forms a 3 ion and how the electron configuratio.
The complete electron configuration for a boron atom will look like this. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10. View the full answer.
Nitrogen He2s 2 2p 3. A S2- b Rb b Au This is the initial text Question 37 1 point Saved 37. Silicon Ne3s 2 3p.
The atomic number of calcium atom is 20.

Boron Electron Configuration Youtube

Boron Electron Configuration Youtube
How To Find The Boron Electron Configuration B

Boron B Electron Configuration And Orbital Diagram

Boron B Electron Configuration And Orbital Diagram
What Is The Electron Configuration Of Sc Quora
Solved Write The Complete Ground State Electron Chegg Com

Solved The Ground State Electron Configuration Of Silver Chegg Com

B 3 Electron Configuration Boron Ion Youtube
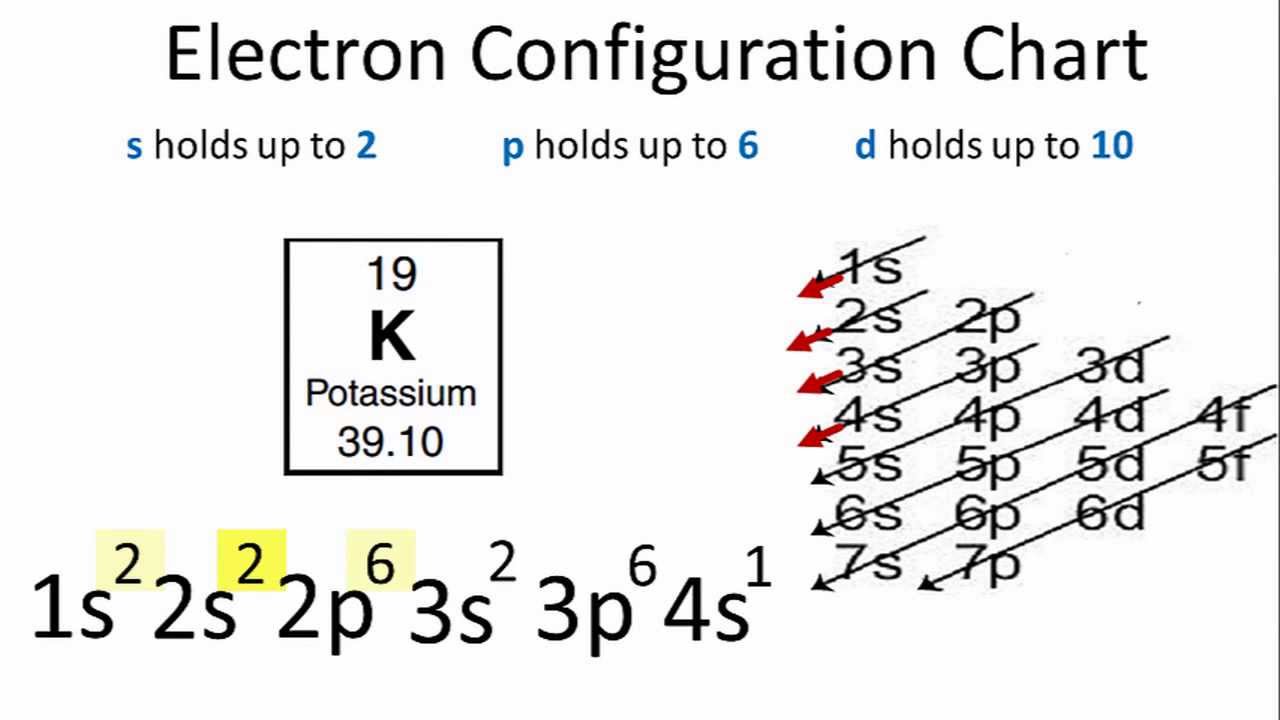
Electron Configuration For Potassium K

Boron B Electron Configuration And Orbital Diagram

Ground State Electron Configurations Of Boron Carbon Nitrogen And Download Scientific Diagram

Electron Configuration For Fe Fe2 And Fe3 Iron And Iron Ions Youtube
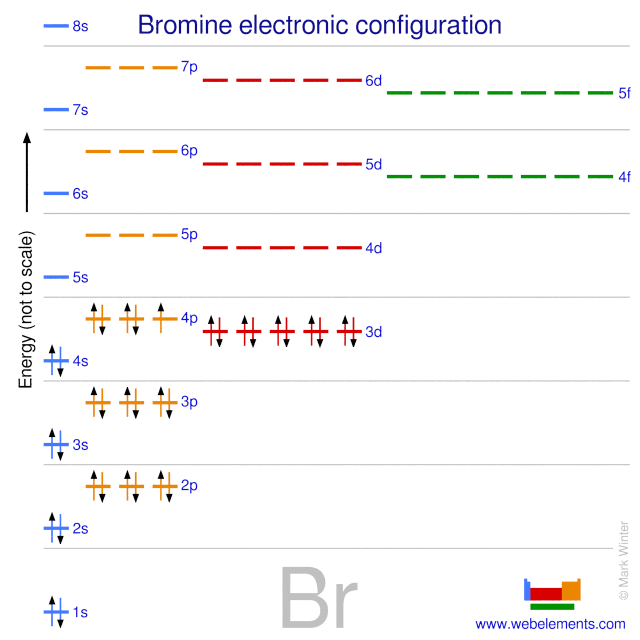
Webelements Periodic Table Bromine Properties Of Free Atoms

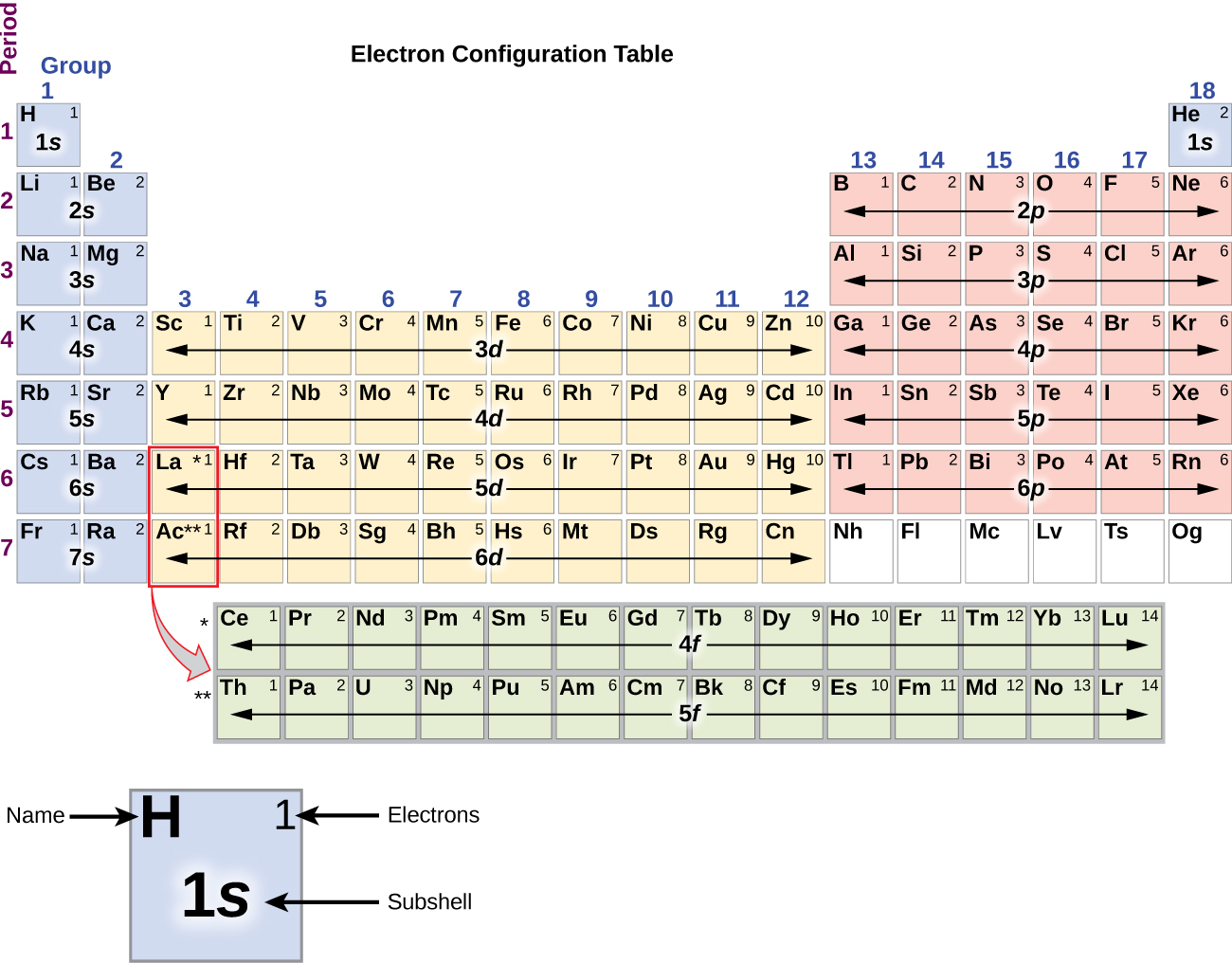
Electron Configurations How To Write Out The S P D F Electronic Arrangements Of Atoms Ions Periodic Table Oxidation States Using Orbital Notation Gce A Level Revision Notes

Chemistry The Central Science Chapter 6 Section 8

Boron B Electron Configuration And Orbital Diagram

Comments
Post a Comment